Introduction
COMORBUSS: The stochastic agent-based viral simulator driven by community dynamics
1 Introduction
COMORBUSS is a computer model for disease propagation due to contacts produced via general social behavior in a community model.
The fundamental objective of any containment policy to curb the spread of a disease spread by contact is to suppress (and ultimately break) the chain of contagion that sustain the viral propagation. Alas, same measures likely produce different results for distinct communities due to different population densities, service structures and social behavior. Therefore, while strict containment policies may be necessary (and sometimes even insufficient) in some cities, the same results may be achieved through less restrictive and damaging measures in smaller communities.
The key to safely navigate long-term the present pandemic is to assess the efficacy of each decision on the viral reproduction in the context of each individual city, so that essential activities are not halted more than necessary (within safety margins). Towards this end, the ModCovid19 collaboration is developing a set of mathematical models and computational tools which will interlink to form a system that will actively warn specific cities on how stringent containment measures must be in each city to sustain its health care system and how each decision taken may contribute to achieve the necessary level of containment.
COMORBUSS (COmmunitary Malady Observer of Reproduction and Behavior via Universal Stochastic Simulations) arises as a components of this systems, aiming at the analysis of the effect of social behavior and containment policies on the reproduction of viruses spread by contact. While the general rate for the propagation of a disease may depend on the absolute size of a community, the reproduction number (the mean number of secondary infections carried out by the vectors of the disease) essentially depends on the mean number of contacts between the population and biological parameters that characterize the spread of the spread of the disease.
We argue that the social behavior around routines and the perusing of service facilities is a main driving factor that produces contacts within a community. We take into account all these different densities and behavioral patterns to generate an universal (i.e. abstract) stochastic model for communities, where individual agents are set to move around their lives along a SEIR model for the disease. The complete model is stochastic in nature, with each individual simulation presenting a possible evolution scenario. All these scenario realizations are used in a Monte Carlo simulation to quantify the effects on behavioral patterns on the viral reproduction.
Fig. 1: simplified representation of social dynamics for the agents in different contexts.
As an example of its capabilities in evaluating the effectiveness of changes in social patterns to the spread of the disease, we present a single realization of the model community
modeled after São Carlos (SP/Brazil):
- 2.500 citizens per km2;
- real age distribution;
- 1 hospital, 3 markets, 2 schools ad 7 restaurants (working at night) per km2;
As a baseline, we present the community driven by simple but unrestricted social dynamics around these services. Next, we declare (in the same realization) that schools and restaurants are closed when 3% of population becomes infectious and that reopening happens when this number drops to 1%; at the same time. In Figure 2 we see that this simplest of measures is effective to "flatten" the infectious curve: though final prevalence of the disease after two months doesn’t change dramatically, the viral reproduction is slowed down to a significant level to protect lives.

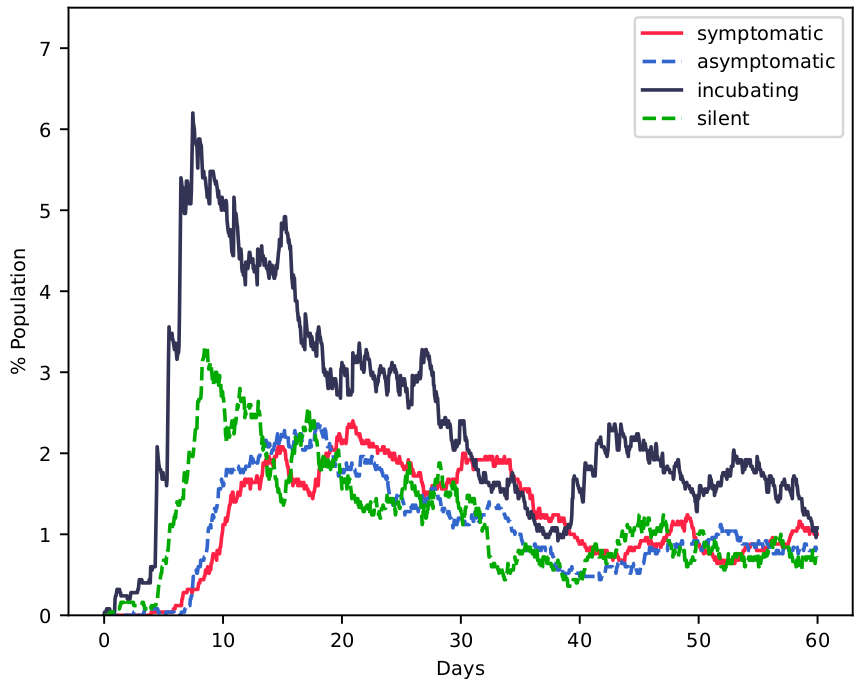
Fig. 2: Fraction of individuals per km 2 in each disease state: incubating, silent (pre-symptomatic), symptomatic and asymptomatic, (Left) with normal activities and (Right) with closing of schools and night restaurants when 3% of population becomes infected (days 5 and 51) with reopening at 1.5% (day 41).
2 Features
COMORBUSS is a detailed agent-based simulator for disease propagation, providing
- clear evaluation on which services/locations contributed most to viral spread;
- which symptomatic states (symptomatic/asymptomatic/silent) proved the most effective vectors for the disease;
- the full tracing of the contact network;
- model diagnostic process and quarantine of patients with positive diagnostics or severe symptoms;
- models for social distancing;
- models for partial or full restrictions on activities and services;
- freedom in choosing criteria to restrain and resume service activities, to simulate and compare different containment measures.
We illustrate a bit of this analytical capability in Figure 3, where in summarize the effectiveness of vectors in different symptomatic states as well as the locations where infections most occurred in the baseline scenario shown previously (where no restriction measures were taken). In this simplistic first example, we suppose that quarantine in hospitals is done perfectly (therefore there are no new infections there).
Already, it is clear that workers in essential services such as markets are likely candidates to become super-spreaders, while intra-domiciliary infections play a big hole in the chain of infections.

Fig. 3: Number of individuals per km 2 in each disease state: incubating, silent (infectious state too early to develop symptoms), symptomatic and asymptomatic. Describes the simulation in Figure 2, (a) with normal activities and (b) with closing of schools and night restaurants when 3% of population becomes infected with reopening at 1% coupled to quarantine of sick individuals.